- Home /
- Impact /
- News & Commentary /
- We Analyzed Public Discussion of Unproven COVID-19 Treatments. Here’s What We Found.
We Analyzed Public Discussion of Unproven COVID-19 Treatments. Here’s What We Found.
Tweets mentioning hydroxychloroquine peaked when President Trump touted the drug — without evidence — as a cure to the disease.
Credit: Judy Zhang
Area of Study
Tags
This article is part of CSMaP’s ongoing Research Summary series. We’ll provide quick, digestible versions of our research articles for peer researchers, journalists, policymakers, and all those interested in the relationship between social media and politics. This week, we wrote about our analysis of Twitter discussion about promising but yet unproven treatments for COVID-19. The article is a preprint and is under peer review.
President Trump first mentioned hydroxychloroquine on March 19, during a Coronavirus Task Force briefing that was broadcast live to millions of Americans.
“I think it could be a game-changer,” he told members of the White House press corps, before clinical trials could establish the drug’s efficacy in treating COVID-19.
In the months leading up to that day, Internet speculation about the anti-malarial drug had spread from China to Vietnam, Nigeria, and France — where the researcher Didier Raoult published findings claiming it had cured most of the COVID-19 patients he and his staff had treated at a facility in Marseilles. That study, since widely debunked, helped bring discussion of hydroxychloroquine to the United States, where it captured the imagination of Trump and the airtime of Fox News hosts who touted the drug as a miracle cure for the contagion sweeping the globe.
People soon panic-bought the drug, which had previously been used to treat not only malaria, but autoimmune diseases like lupus and rheumatoid arthritis. The prescription rate in New York alone rose by more than 40 times the average, with more than 40,000 healthcare workers becoming first-time prescribers of the drug in March.
Fast-forward to July. Scientists and regulators expressed concern over the drug’s side effects, which include sudden cardiac death. People with autoimmune conditions feared disease flares due to medication shortages. And the Food and Drug Administration revoked its clearance for the drug to be prescribed off-label on an emergency basis, citing a clinical trial showing no benefit to COVID-19 patients.
We wondered: Is there a gap between public attention and discussion around COVID-19 treatments and the scientific evidence behind their safety and efficacy? And if so, can we observe trends in these public discussions on social media platforms and equip public health officials with the necessary information to close the gap?
Our researchers ran a study to answer these questions. We analyzed discussion on Twitter about three exploratory treatments during the early days of the pandemic: hydroxychloroquine, the antiviral drug remdesivir, and antibody-rich blood from people who recovered from COVID-19, known as convalescent plasma. The results were sobering: Most of the information about these treatments that reached Twitter users came from ideological sources. In many cases, these sources spun scientific results to suit a political agenda.
“Hydroxychloroquine could be one of the most politicized drugs of all time,” said Tymor Hamamsy, a Ph.D. student at NYU’s Center for Data Science who co-authored the study with Richard Bonneau, professor of biology and computer science and co-director of NYU’s Center for Social Media and Politics.
Most of the information about these treatments that reached Twitter users came from ideological sources. In many cases, these sources spun scientific results to suit a political agenda.
Hamamsy noted a troubling divide along party lines among the members of Congress who tweeted about hydroxychloroquine, with Republicans responding positively to the drug on Twitter and Democrats responding negatively. “It’s a drug, so it’s surprising to see differences along political ideology,” he said, adding that politicians’ sentiments toward COVID-19 treatments should be informed by scientific evidence, not politics.
Here’s how we arrived at our results: Using Twitter’s public API, we screened for tweets mentioning the three treatments that were posted between February 28 and May 22, 2020. We calculated the number of tweets and retweets mentioning each treatment per day during this time window — and found more than 440,000 tweets containing “hydroxychloroquine” or “hcq,” more than 60,000 containing “remdesivir,” and nearly 64,000 containing “convalescent” or “plasma.”
We then performed a “sentiment analysis” on the tweets using a lexicon designed to interpret and classify emotions in text data. We also analyzed the URLs shared via these tweets, counting how often different websites were shared and searching for mentions of journal articles or preprints. Finally, we searched hydroxychloroquine tweets for mentions of adverse drug reactions. We then visualized all data alongside major announcements about treatments, clinical trials, and the government’s response to the pandemic.
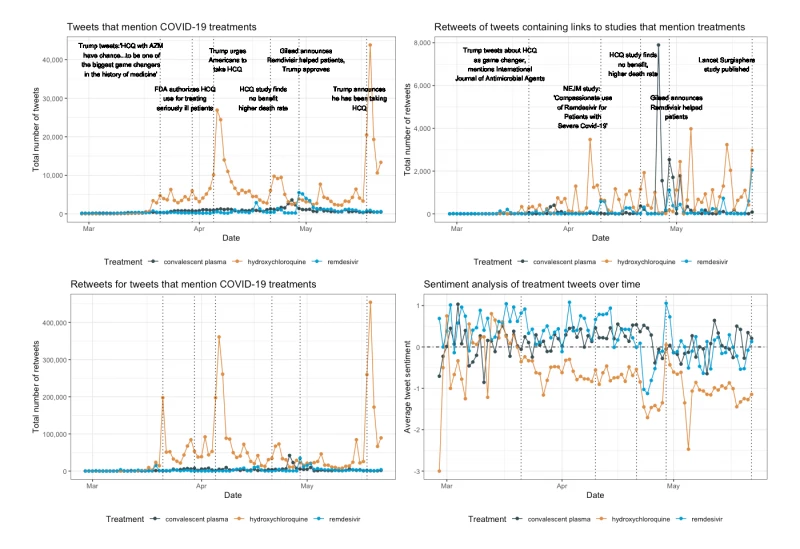
Hamamsy said trends in the data “align very well” with noteworthy events and announcements around COVID-19 treatments. Tweets about hydroxychloroquine, for example, peaked three times after Trump promoted the drug on March 21, April 5, and May 18, with 31 percent of these tweets mentioning his name (see Figure 1).
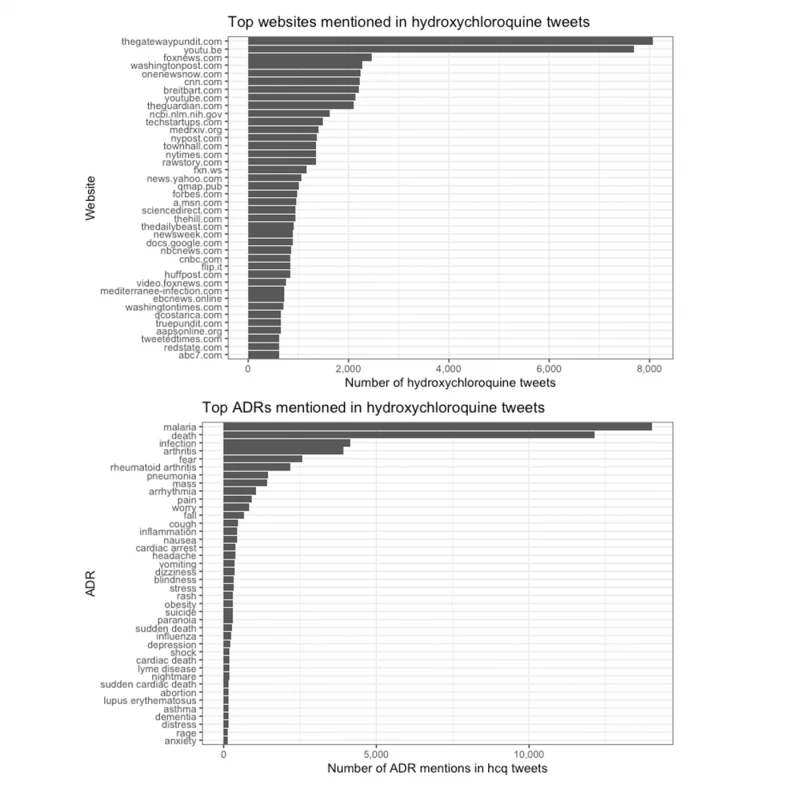
We found further evidence of the politicization of COVID-19 treatments in our analysis of links shared via tweets: The top websites mentioned in tweets about hydroxychloroquine leaned conservative and even far-right. These included Fox News, Breitbart News, and the Gateway Pundit (see Figure 2).
Our sentiment analysis reflected polarized attitudes about treatments: We found 72 relevant tweets from members of Congress, who were split along party lines in their evaluation of hydroxychloroquine. Average sentiment was negative among Democrats (-.41) and positive among Republicans (.88).
The bottom line? We found a disconnect between the scientific evidence behind experimental COVID-19 treatments and public discussion of these treatments on social media. And we believe our research design offers a starting point for public health researchers to build on as they seek to learn more about the problem.
Hamamsy noted the relative novelty of social media discussion at this pace and scale during a pandemic. He said it offers a useful data stream for the FDA, the Centers for Disease Control and Prevention, and other stakeholders interested in public health surveillance — especially as COVID-19 vaccine trials get underway.
“It will be critical to research these public discussions when vaccines are on the horizon,” he said.
Read the Original Medium Post Here.